An automobile is a type of rechargeable battery that’s used to start a vehicle’s engine. Its primary function is to provide an electric current for the electric-powered starting motor. This current then activates the vehicle’s internal combustion engine, which produces a chemical-based energy to propel the car. After the engine is powered up, the battery’s electrical systems are still charged.
An automobile battery is a storage and generation electrochemical device (one that requires both electrical and chemical energy to function). Due to the materials used, a standard car battery is also known as a lead-acid battery.
The term “battery” refers to the collection of electrically active cells that make up a volt battery. Putting two different metal electrodes (plates that can give and take electrons) in an acid bath creates a basic battery cell. An electric current is created when electrons are pushed away from one electrode and drawn toward the other. Check out the diagram in Figure 2.
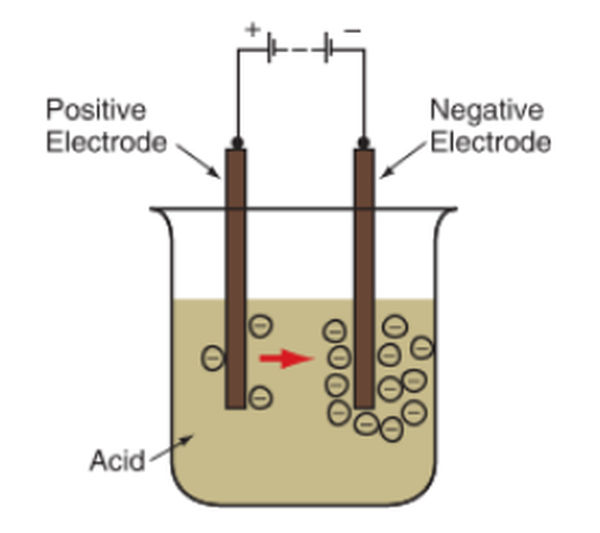
Figure 2. A basic battery cell can be made by placing two unlike metal electrodes in a jar of sulfuric acid. A chemical reaction occurs in which electrons flow between the two electrodes.
Battery Cell Construction
The parts of a basic battery cell include:
Positive plates—electrodes of lead peroxide (PbO2).
Negative plates—electrodes of finely ground or powdered lead (Pb).
Electrolyte—a solution of sulfuric acid (H2SO4) and water (H2O).
Separators—material that keeps the positive and negative plates from touching and creating a short circuit.
The plates in batteries have a porous lead alloy coating over a rigid mesh grid. See 3A in Figure. Sponge lead is the chemically active material in the negative plates (lead that has been finely ground or powdered to increase its porosity).
Lead on the plates is porous, so the sulfuric acid can soak in there like a sponge. The chemical reaction and electricity generation benefit from this.
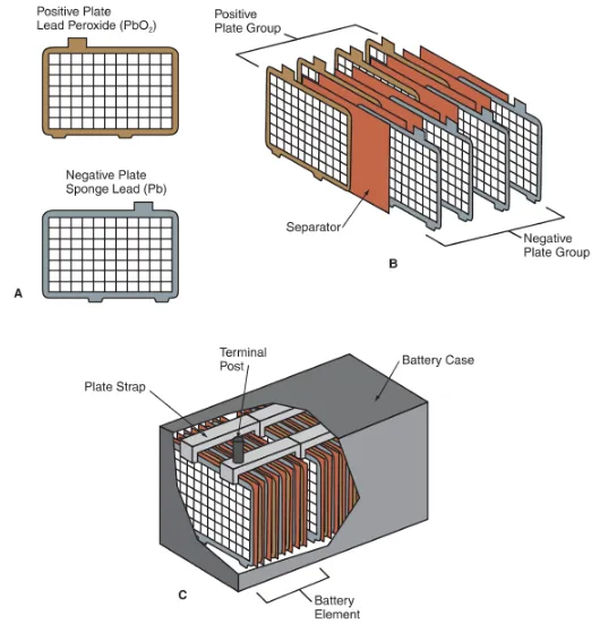
A-Plates, both positive and negative. Take note of how the tabs are positioned along the top edge. These prongs make electrical contact with the plate strips.
B—Separators prevent the plates in the positive and negative plate groups from touching and causing a short circuit.
C – Each component of the battery is held together by a plate strap. The electrical current is transferred from one battery to another via the plate straps, which are connected end to end. Posts for the ends are typically shaped from the plate straps themselves.
Positive plates use lead peroxide as the active material. As a means of boosting battery performance and lowering gassing, lead is sometimes combined with calcium or antimony (the formation of explosive hydrogen gas during the chemical reaction).
Each cell requires multiple battery plates to produce sufficient energy for automotive use. See 3B in Figure. Several negative plates are joined into one large negative plate by using a metal plate strap to hold them all together. One more plate strap connects the plates that are positive together to form a positive plate cluster.
Each cell’s connectors must make electrical contact with those of neighboring cells for power to flow continuously along the battery’s length. Please refer to 3C in the figure. End connectors that double as battery terminals (posts or side terminals) are a standard feature.
To prevent the battery plates from shorting out to each other, separators are placed in between them. The separators are permeable, so the electrolyte can flow freely around the plates.
Battery elements include the negative plate group, positive plate group, plate straps, and their separators, while battery cells include these parts as well as electrolyte. Read back Figure 3C.
Sulfuric acid and distilled water make up the electrolyte in a car battery, also known as battery acid. The final step in making a fully functional battery cell is to pour this mixture into each one until the plates are covered.
Battery Case
The battery cells are contained within the battery case of a car. In most cases, premium polypropylene is used in its production. Battery acid can be corrosive, so the case needs to be sturdy enough to withstand extreme temperatures, sudden temperature swings, engine heat, and vibration. The case’s dividers create separate compartments for each cell.
The top of the battery case is bonded to the cover. It prevents water from entering the case from the top and has slots for battery caps or cell covers directly above each battery. View Figure 4 if you don’t believe me.
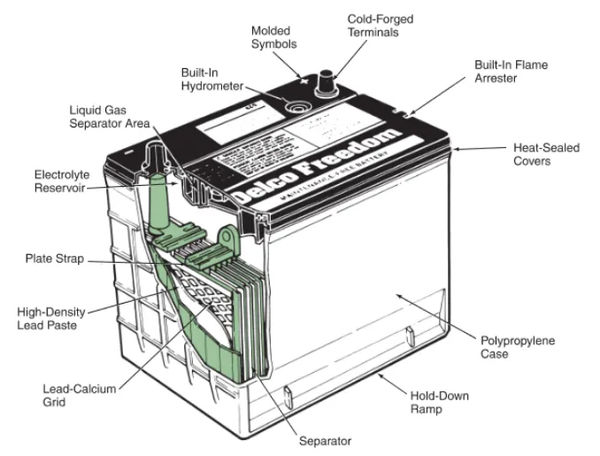
Automotive Battery Terminals
Terminals for the battery allow the plates to be connected to the car’s electrical system. Typically, they are made integral to the terminal connectors. Two terminals, such as those on the ends of batteries, can be used. Figure 5 depicts the threaded posts used on some large truck batteries.
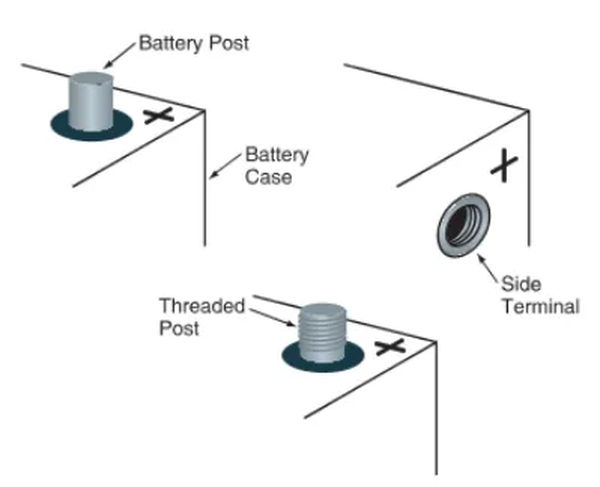
Posts are the circular metal terminals that protrude from the top of a battery case. A male end fits into a female connector on a battery cable. Size-wise, the positive pillar is more noticeable than the negative one. It could be labeled with a crimson dot and a plus sign (+). The smaller, potentially black or green negative post. Typically, you’ll find a minus sign (-) on or near the item.
The battery’s side terminals are its electrical connections. Female threads allow them to receive a dedicated bolt at the end of the battery cable. The case has symbols for positive and negative polarity that indicate which terminals should be connected to which sides.
Automotive Battery Charge Indicator
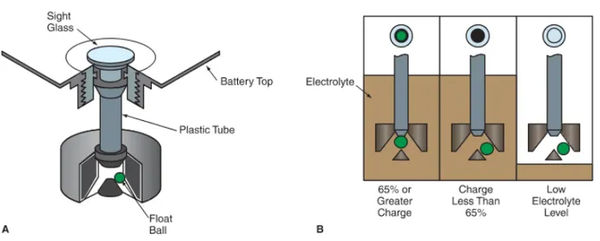
A battery charge indicator, also known as a hydrometer, determines the battery’s state of charge by measuring the specific gravity of the electrolyte. You are hereby reminded to look at Figure 4 once more.
A solution’s specific gravity is its density compared to that of water (1.00). The electrolyte’s specific gravity approaches 1 as the battery loses its charge.
As can be seen in Figure 6, the charge indicator displays a different color depending on the battery’s charge level. If the battery is fully charged, the indicator light might turn green. When the battery is dead, it may turn black, and when it’s time to replace it, it may turn yellow.
Battery Tray and Retainer
The battery is safely fastened in place by a tray and a retainer. As the vehicle moves, the battery is protected from vibrations by these. The battery can be damaged if the tray and retainer are not in good working order and are loose. Observe the graphic in Figure 7.

Automotive Battery Cables
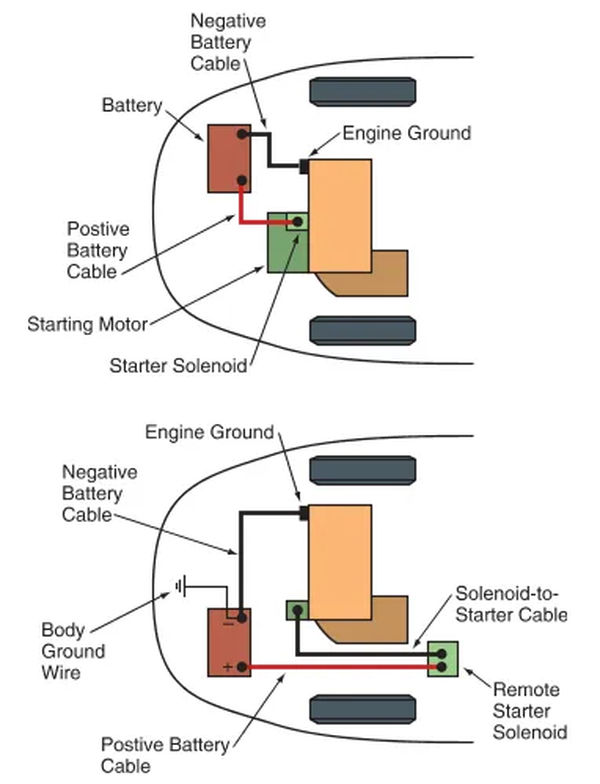
Large conductors called battery cables link the battery terminals to the car’s electrical system. As shown in Figure 8A. The starter solenoid is connected to the positive, or battery, cable, which is typically red. It is common practice to attach the negative (black) battery cable to the engine block’s ground terminal.
As can be seen in Figure 8B, some vehicles’ negative battery cables also include a body ground wire to ensure the vehicle body is properly grounded. It is possible that a component grounded to the vehicle’s body will not function if this wire does not make a good connection.
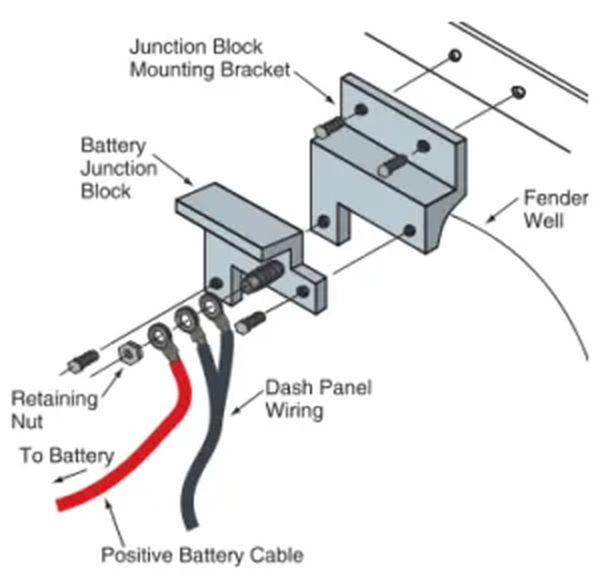
Between the positive battery terminal and the solenoid, a battery junction block is sometimes installed. This junction block facilitates the connection of additional wires to the positive battery cable, thereby supplying power to the instrument panel and other accessories. Look at Figure 9. The starter solenoid in some vehicles may perform a similar function.